Shape memory alloys (SMAs) are materials that can be deformed at one temperature but when heated or cooled, return to their original shape, i.e. the alloy appears to have a memory. Strains of up to about 10% can be fully recovered. Alloys that exhibit this effect only when heated are said to have a “one-way shape memory”. This transformation sequence is illustrated below.
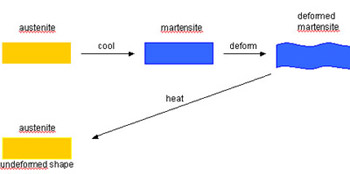
One-way Shape Memory Effect
Some materials also show a memory effect on subsequent cooling and these are said to have a “two-way shape memory” but for these materials the recoverable strain is limited to about 3%.
SMAs are particularly useful because at the lower temperature, they have a rubbery feel and can be deformed by a small force; at the higher temperature, they behave like normal metals and any induced strain is not recoverable.
SMAs also exhibit superelasticity (or pseudo elasticity). In this case, a small force induces considerable deformation but when the force is removed, the material automatically recovers its original shape without the need for heating.
Products containing SMAs have been around for many years but consumers are often unaware of their presence because they are usually buried in the mechanism that controls the function of the product. One of the few overt applications is in ‘indestructible’ spectacle frames; these can be bent and twisted to a remarkable extent and then regain their original shape.
The Mechanisms of the Shape Memory Effect and Superelasticity
Shape Memory Effect
SMAs are said to exhibit a crystallographically reversible martensitic transformation. At high temperatures, the SMA exists as an austenite phase (the parent or memory phase) with long range order. On cooling below the transformation temperature, the austenite transforms to a thermoelastic martensite whose structure has many variants, typically sheared platelets. Because the martensitic structure is self-accommodating, the deformation on transformation to martensite is zero. The martensite deforms by a twinning mechanism that transforms the different variants to the variant that can accommodate the maximum elongation in the direction of the applied force. The interfaces between platelets in the martensite phase slip very readily and the material is deformed at low applied stresses. The austenite phase has only one possible orientation, thus when heated, all the possible deformed structures of the martensite phase must revert to this one orientation of the austenite memory phase and the material recovers its original shape.
The transformation temperature is a function of the alloy type, composition and also of the thermomechanical treatments applied. Alloys with transformation temperatures below -100C and above 150C are known. The heating and cooling transformations do not overlap and the transformation is said to exhibit hysteresis. The magnitude of the hysteresis also varies with the alloy type and is typically in the range 10-50C. A typical transformation is illustrated below where a physical property of the alloy, e.g. electrical resistance, is monitored to follow the phase transformations.
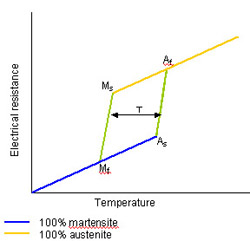
M s Temperature at which martensite starts to form
M f Temperature at which conversion to martensite is complete
A s Temperature at which austenite starts to form
A f Temperature at which conversion to austenite is complete
T Transformation hysteresis
Superelasticity
SMAs also display superelasticity that is a mechanical type of shape memory. This effect is observed when alloys are strained just above their transformation temperature.
The mechanical properties of SMAs vary over the temperature range spanning their transformation. At low temperatures, the material exists as martensite and is deformed by a relatively small applied force; it also exhibits shape memory on heating. At high temperatures, the material exists as austenite, which is not easily deformed, and, on heating, no shape memory occurs because there is no phase change.
However, if the material is tested just above its transformation temperature to austenite, the applied stress transforms the austenite to martensite and the material exhibits increasing strain at constant applied stress, i.e. considerable deformation occurs for a relatively small applied stress. When the stress is removed, the martensite reverts to austenite and the material recovers its original shape. This effect, which makes the alloy appear extremely elastic, is known as superelasticity or pseudoelasticity.
Shape Memory Alloy Systems
The shape memory effect was first observed in AuCd in 1951 and since then it has been observed in numerous other alloy systems. However, only the NiTi alloys and some copper-based alloys have so far been suitable for commercial exploitation.
Copper Alloy SMAs
Copper-Zinc-Aluminum (CuZnAl)
CuZnAl was the first copper based SMA to be commercially exploited and the alloys typically contain 15-30 wt% Zn and 3-7 wt% Al. The useful transformation temperature for this system ranges from -100C to +100C; the actual transformation temperature is a function of both the alloy composition and the thermomechanical treatments applied during its manufacture. The major advantage of the CuZnAl alloys is that they are made from relatively inexpensive metals by conventional metallurgical processes which makes them the cheapest of the commercial SMAs. However, their memory properties are modest with a maximum recoverable strain of about 5%. The ternary alloys also have a very large grain size which makes them brittle but the addition of < 1% of a grain refiner such as titanium or zirconium, limits the grain size and solves the brittleness problem. The major disadvantages of this alloy system are that the martensitic phase is stabilized by long term aging even at room temperature causing an increase of the transformation temperature, and the alloy structure decomposes when exposed to temperatures above 100C. These disadvantages have more than outweighed the cost advantage of the CuZnAl alloys and this alloy system is rarely used today.
Copper-Aluminum
The binary alloy has a transformation temperature that is far too high for practical applications and a third element is usually added to produce a useful alloy. Copper-aluminum-nickel (CuAlNi) alloys have undergone extensive development and are now preferred to the CuZnAl alloys. The alloys typically contain 11-14.5% Al and 3-5% Ni and have transformation temperatures in the range 80-200C dependant on their composition, the transformation temperature is particularly sensitive to the aluminum content.
The alloy is again made from relatively inexpensive elements but its processing is more difficult since it can only be hot worked and the final heat treatment has to be tightly controlled to produce an alloy with the desired transformation temperature. These processing difficulties have made this alloy system more expensive than CuZnAl but it is still less expensive than NiTi. The ternary alloy Cu13Al4Ni is one that is often used commercially.
Some improvement of the mechanical properties can be obtained by reducing the aluminum content below 12%, adding 2% manganese to reduce the transformation temperature and 1% titanium as a grain refiner but these additions can affect the stability of the alloy structure.
The major advantages of the CuAlNi system are its wide range of useful transformation temperatures, its stability at elevated temperature making it the only system that can be used for applications above 100C, its small hysteresis and its relatively low cost.
A new alloy, where the nickel is replaced by beryllium, has recently achieved commercial acceptance. Cu12Al doped with less than 0.5% of beryllium extends the transformation temperature range from 100C to -200C. This CuAlBe alloy exhibits excellent superelastic and damping properties.
Nickel-Titanium Alloys (Niti)
This system is based on the equiatomic compound of nickel and titanium; it can tolerate quite large amounts of shape memory strain, is very stable and corrosion resistant. The transformation temperature is very sensitive to the composition and small changes of the nickel content cause large changes. The system also has a large transformation temperature hysteresis, typically about 50C.
For commercial exploitation, a third metal is usually added to the binary alloy to improve its properties. Up to 30% of the nickel can be replaced by copper without loss of the shape memory effect. The effect of the copper addition is to reduce the hysteresis to about 15C and also make the transformation temperature less sensitive to changes of the nickel content. Copper shows most benefit at concentrations up to about 10% and further additions only produce marginal improvements
Manufacture of the NiTi alloy is difficult because of the reactivity of titanium and all melting must be done in vacuum or in an inert atmosphere. Joining the alloy by welding, brazing or soldering is also difficult for similar reasons. When cold worked, the alloy work hardens very quickly but it has a very fine grain structure and fine wire has been successfully produced. Many machining techniques can only be used with difficulty with NiTi alloys. The alloy is therefore very expensive and costly to fabricate and use. Despite these disadvantages, the excellent shape memory properties and its corrosion resistance have resulted in NiTi being used in a wide variety of applications. In recent years, biomedical applications have been a major growth area where both the memory and superelastic properties of NiTi are exploited.
Applications of Shape Memory and Superelastic Alloys
There are several thousand patents for devices utilizing the properties of SMAs. Only a small percentage of these inventions have become successful products but the fields of application are very diverse ranging from everyday consumer products to biomedical implants to space applications. Only a few of these applications can be mentioned here.
The first industrial application occurred in 1969 when SMA couplings joined hydraulic pipes in the F-14 aircraft. This application has been extended to the joining of many other types of pipe, sometimes using a liner that is squeezed onto the pipes to make a joint. The cylindrical couplings are cooled to cryogenic temperatures to produce the martensitic phase when they can be easily expanded to slide over the pipes. On warming above the transformation temperature, the coupling tries to contract to its original size but is constrained by the pipes within. The stresses that result from this constraint are sufficient to create a joint that can be superior to a weld.
One obvious field of application is in devices to protect against fire. Fire sprinkler systems can be activated by the shape change induced by the heating of an SMA in a fire. Similarly, a fire safety valve incorporating an SMA activator shuts off the flow of a flammable or toxic gas if a fire occurs. A construction application uses an SMA actuator to lock ceiling plates in place if the temperature rises above 60C protecting pipes, cables and the floor above from the effects of the fire.
SMAs can also be used as an improved bimetallic strip to regulate water temperature. An anti-scald device in a shower head introduces cold water if the water temperature becomes too high. This application uses an SMA compression spring and a biasing steel spring. At high temperatures, the SMA spring expands and opens a needle valve allowing cold water to enter the mixing chamber. When the temperature is reduced, the SMA returns to martensite and the steel spring resets the shape memory spring and simultaneously closes the needle valve.
A domestic deep fat fryer uses an SMA blade to prevent the basket being lowered into the oil until the correct temperature of 170C has been attained. This high temperature application favors the use of a CuAlNi alloy. Similarly, CuAlNi alloys are preferred for circuit breakers to prevent overload electric currents heating wires and cables above 140C.
Superelastic SMAs are used in the frames of indestructible spectacles. Superelastic wire also makes excellent springs. Many biomedical applications use superelastic wires and tubes. These include catheters and guide wires for steering catheters. Superelastic arch wires for orthodontic correction have proven particularly effective by producing large rapid movement of teeth.
A future application for SMAs that may become extremely important is in ‘Smart Materials’. Many structures are designed with reinforcements and backup systems to provide for the worst case scenario. These structures therefore use more materials and energy than is required for normal use. Smart materials would sense their environment and modify their behavior in extreme circumstances thus avoiding the need for reinforcement or backup systems. Smart materials would be composite structures with embedded sensors and actuators. Thus the concrete infrastructure of a bridge could contain sensors looking for cracks or corrosion and embedded SMA actuators would counteract the strain induced by this degradation. Similarly, an aircraft body could contain a thin layer of sensors that would monitor subtle physical and chemical changes associated with fatigue and actuate a layer of SMA to compensate for these changes and prevent failure.

